
 GEOLOGICAL SCIENCES
GEOLOGICAL SCIENCES

RESEARCH PROJECTS
EXPERIMENTAL AND THEORETICAL AQUEOUS GEOCHEMISTRY
Projects involve application of computer modeling to problems in fluid-rock interaction and the analysis of the mechanisms by which progressive metamorphic reactions occur. Volatile production, transport, and fluid-rock ratios have been evaluated (Wood and Walther, 1986). The calculated rates of surface reaction (Wood and Walther, 1983; Walther and Wood, 1986) and the controls on the rate-limiting step in metamorphic reactions have been analyzed (Walther and Wood, 1984).
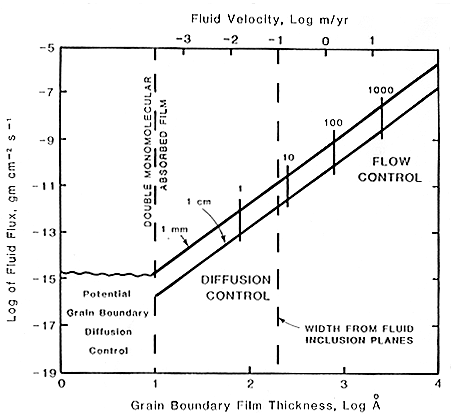
Diagram showing when aqueous diffusion controls the transport of material between reactant grains and when flow controls transport for 1 mm and 1 cm average grain size for a prograde metamorphic reaction. Fluid velocity of the intergranular film is shown at the top of the diagram. The short vertical lines labeled 1, 10, 100 and 1,000 show the boundary between aqueous film diffusion and flow transport control for these values of the chemical potential gradient of the least soluble mobile component in calories per mole per cm. At very low fluid fluxes the fluid film drops below a double monomolecular absorbed film and true grain boundary diffusion control can occur. (Walther and Wood, 1984).
KINETICS OF DISSOLUTION AND FLUID-MINERAL SURFACE INTERACTIONS
Research is being carried out to study the rates and mechanism by which minerals dissolve in order to understand the controls on the composition of surface waters and the role of pH, salts and organic acids:
MINERAL SOLUBILITIES AS A FUNCTION OF TEMPERATURE, PRESSURE AND SOLUTION COMPOSITION
A continuing program in the laboratory is the measurement of solubility of minerals in aqueous solutions to determine the thermodynamic properties of species to temperatures of 650 degrees C and pressures of 3 kbar (e.g., Walther, 1986). Solubility information is necessary to help determine the way in which minerals react to equilibrium, the textures that are observed, and the extent of veining and mass transfer in geological processes. Recent and current projects including those with students:
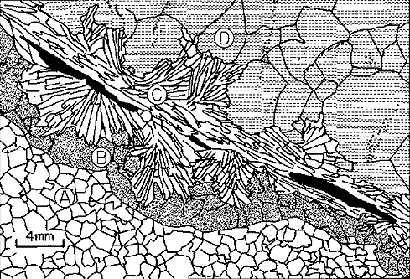
Drawing after a photomicrograph of reactions zones developing between dolomite = (A) and quartz = (D) to form calcite = (B) and tremolite = (C). Shaded areas within tremolite show the location of phlogopite. Dots within quartz crystals show the orientation of fluid inclusion planes along healed micro-fractures (Walther, 1983).
|
Dr. John V. Walther Department of Geological Sciences Southern Methodist University Dallas, Texas 75275-0395 voice: (214) 768-3174 fax: (214) 768-2701 email: walther@mail.smu.edu |
|---|


|

|
|

|

|

|

|

|